ZIM-INTERNATIONAL INNOVATION NETWORK
4D Bioprinting



MOTIVATION
The need for human organs and tissues is a major factor in driving developments in 3D bioprinting. However, the path to complete, additively printed organs for transplantation medicine is still long. An upcoming achievement is the 3D printing of tissues produced with human cells, which are viable and capable of complete metabolism. They can be used in drug research since they are closer to human metabolism than experimental animals and would therefore have the potential to achieve results that could be promptly transferred to humans while simultaneously reducing the number of test animals required.
3D bioprinting is currently used for a wide range of applications in the pharmaceutical and biomedical sectors. However, the printed end products are static and not alive. Bioprinting of viable cells involves time as a fourth dimension, hence “4D Bioprinting”, whereby the functionality and shape of the printed objects evolves over time. This includes a change in the cells due to biochemical environment, temperature, light irradiation, and other parameters which lead to fundamentally new technical challenges.
VISION
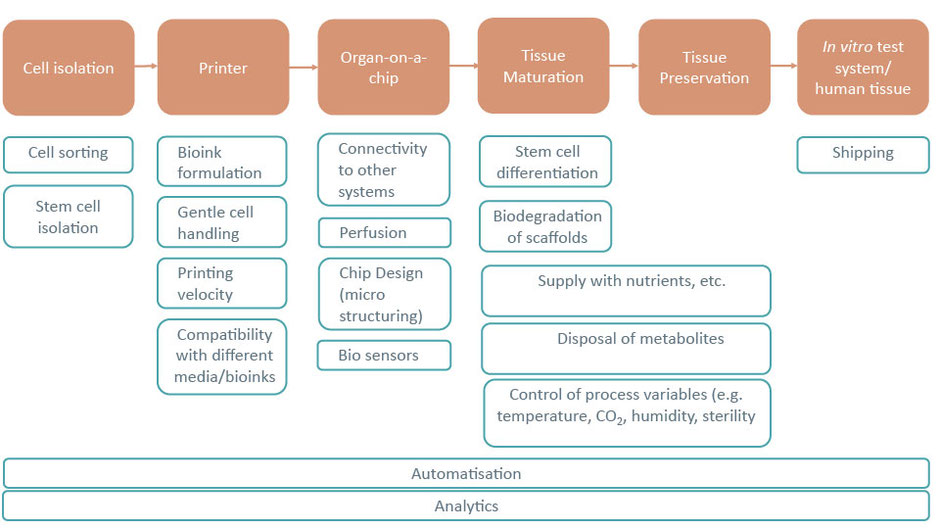
The vision of the network is the development of interdisciplinary and innovative solutions and products along the entire process chain from cell isolation to a printed end-product, such as an in vitro test system for drug development or tissue for direct use in the human body.
The network will bundle the different competencies of the network partners and connect users and developers to enhance the development of innovative and interdisciplinary solutions and products.
AIMS
The aim of the network is the initiation, concept development, and funding advice for research and development projects of network partners. The projects will substantially enhance 4D bioprinting techniques, accelerate technological transfer, and therefore contribute to improved medical care.
CONTENT
The network addresses small and medium enterprises and research institutes that aim to develop products or processes along the above-described process chain.
The planned R&D projects and other network activities, including user workshops or participation in trade fairs, congresses, and events, will increase know-how amongst the network participants and thereby contribute to their competitive advantage.
ABOUT US
PARTNERS
Our network partners are small and medium-sized companies and research institutions from all over Europe.
MEMBERSHIP
Joining forces in a cooperation network brings numerous advantages for your company. You can also find out here which services the network management takes over for you
NETWORKMANAGEMENT
The network management introduces itself. Here you will find the right contact person.